Autoradiography of Receptors: In vivo and in vitro labeling
Updated February 11, 2011
Abstract: Because it was possible to inject animals with radioactive drugs that had high affinity to receptors, and thereby label the receptors in vivo, it was possible to localize drug receptors at the light microscopic level by autoradiography. This was the basis for the future mapping of receptors by PET scanning. An important extension of the work is in vitro labeling autoradiography. In this approach, the receptors are not labeled with radioactive drugs in vivo, but rather in vitro by incubations of slide mounted tissue sections with radioactive drugs that had high affinity to specific receptors. Overall, these receptor mapping studies were well received and have become standard techniques in neuroscience. The scientists who developed the techniques are discussed.
Yours truly at his favorite microscope at Johns Hopkins in about 1983.
In vivo labeling of receptors and autoradiography
This story begins in the early 1970s and describes how I worked out and adapted techniques so that receptors could be localized at the light microscopic level by autoradiography. The setting was our neuropharmacology group at Johns Hopkins lead by Sol Snyder who had mastered the techniques of identifying new receptors by radioligand binding in tissue homogenates. The approach was to select a drug that was both specific for a given receptor and very potent, indicating a high affinity for the receptor. This drug in a radiolabeled form would bind to the receptor in tissue fragments on filters, and nonspecific (non receptor) binding could be washed away by gentle rinsing of the tissue laden filter papers. This in vitro binding approach was very successful because the conditions for binding could be manipulated and selected so that non specific binding was very low. This identification of receptors by binding techniques was a major breakthrough and offered drug companies, for example, at that time, a new approach in screening for drugs.
Receptors could be measured in specific brain regions by dissecting the regions and then carrying out binding in homogenates of that region. But by this approach you could only measure receptors in as small a region as you could dissect. To measure receptors in smaller and smaller anatomical regions, a light microscopic approach was needed. But a problem was that the drug binding was reversible from the receptors, so the techniques to be used had to minimize diffusion of drug from binding site.
Now here is where some serendipity comes in. I did my post doctoral training at Yale with Drs Bob Roth and George Aghajanian. George, near the end of my stay there, told me about a frozen section autoradiographic technique that would reduce diffusion of drug. Some of our colleagues, when it became obvious that we should try to localize receptors with the microscope, recommended the same technique! The technique was developed by Lloyd Roth and colleagues at the University of Chicago, as well as others, and was practiced by various investigators in the field. Bruce McEwen at Rockefeller was doing it and I went there on Oct 9, 1973 to see the procedure done. I brought it back to Hopkins and assessed how to go forward.
Sol Snyder and his fellows were injecting high affinity radioligands (drugs) into rats and showing that under certain condition of time and dose, most of the radioactivity in the brain was drug bound to receptors. Hank Yamamura from Sol’s lab and I decided to try this and localize the receptors by autoradiography at the light microscopic level. We injected rats with high specific activity QNB, which labeled cholinergic muscarinic receptors very selectively. Then the brain was frozen so that the tissue would be intact and so that there was no opportunity for the radiolabeled drug to diffuse away from the receptor. The next step was to section the brains in a cryostat under freezing conditions so that thin frozen sections could be transferred to emulsion coated slides. Of course this had to be done in the dark so that the emulsion wasn’t exposed by the light. It was the radioactivity in the tissue sections that produced exposed grains in the emulsion. Because the radioactive molecules were linked to the receptors, and because the emulsion revealed where the radioactivity was, then the emulsion showed the location of the receptors as well.
Hank Yamamura and I injected rats with the radioactive drug (QNB) on 12/20/1973, and then sectioned the brains and placed the sections on dry emulsion coated slides on 1/8/1974. On February 11, the emulsion was developed and we saw, the distribution of cholinergic muscarinc receptors in rat brain! The photo log book indicates that photos for publication were taken on Mar 11, 1974. We were successful! We were now ready to study additional receptors. I did these autoradiographic experiments 99% by myself and was first author on the publications. On May 1 we sent the abstract to the Society for Neuroscience, and on Aug 8, 1974 submitted the paper to Science, who unfortunately rejected it. But Nature accepted it for publication on Jan 7, 1975 (253:560-561, 1975). It took more time to get these first papers on muscarinic receptors published because we were working out procedures and controls. Subsequent studies of other receptors used that technology and were done much faster. Naomi Taylor, who was a super technician, helped a lot in the darkroom, and we have reminisced over these experiments many times.
Now being sure of successes with the technique, we then moved on to mapping the brain for opiate receptors with Sol and Candace Pert. Radioactive diprenorphine was used to label the opiate receptors in brain. The injections were made around Nov 1, 1974, almost a year after we injected rats with QNB for muscarinic receptors. The first slides were developed on 11/25/1974, and photos taken over the next several months. I still have the slides and photo logbook which is fun to look at. Some of these photos were shown at a the Airlie House meeting on opiates on May 21, 1975, and they were a big hit! There was a rapid publication in Life Science (16:1849-1854,1975); The more detailed paper was accepted for PNAS by Dr Vernon Mountcastle. I have a letter dated June 24, 1976, indicating that it was a go for publication in PNAS.
The autoradiography of receptors after in vivo injection of radioligand was now firmly established in our hands. But it wasn’t simple. It took quite a bit of practice and experimental skill to do it well. Many students and fellows were not able to get aspects of the technique done well and underestimated how much work and practice it took. I remember throwing out entire experiments done by others, because the tissue sections weren’t flat, for example. I had to repeat much of the work on the opiate receptors. Sometimes memories are faulty, but the original notebooks are very clear.
Importantly, this approach was the basis for positron emission tomography (PET) scanning of receptors. Using injections of drug in vivo followed by autoradiography, we were we able to provide solid evidence that we were “visualizing” receptors with autoradiography. Later, we would “visualize” receptors with PET – a noninvasive technique!
In vitro labeling of receptors and autoradiography
The next advance came a few years later, in the late 1970s. At this point, we could “visualize” receptors by autoradiography after injecting the drug into an animal under certain conditions of time, dose and specific activity. But this was limited to the drugs that in fact bound tightly to receptors in vivo. But not all did. In fact, most ligands did not. So if we wanted to do more receptors with more ligands, and if we wanted to use post mortem human tissue (PET scanning of humans was not yet available), we would need to expand out techniques.
If we could take slide mounted tissue sections (from untreated rats), and incubate them with radiolabeled drugs in vitro, then we could manipulate the conditions of incubation to get better labeling of receptors. Moreover, we needed a procedure that worked with reversible drugs because most receptor binding was carried out with reversible ligands (ligand = the drug that bound to receptors).
Again the work of Lloyd Roth and others was a guide. Dry emulsion-coated coverslips were used to record the binding sites of radiolabeled drugs in tissue sections, and we adopted this approach. The tissue sections with radiolabeled receptors were placed against dry emulsion (“dry” so that the radioactive drug wouldn’t diffuse). In theory, this was feasible, but we didn’t know if it would actually work.
A graduate student, Scott Young, decided that he would use the topic for his PhD dissertation. I mentioned above how difficult some of the procedures were, but Scott persisted and it took about 18 months to get it all worked out. We did receptor binding in intact tissue sections mounted on microscope slides, and it was remarkable to us at the time, that it worked. All of the characteristics of receptor binding on slides were basically identical with the characteristics of binding in homogenates, the accepted method. We showed this by incubating the slide mounted sections with ligands and then wiping the tissue off the slides and measuring the radioactive binding. Having proved to ourselves that the radioactivity on the slides was on receptors, we then used the dry coverslip technique to localize the receptors (Brain Res, vol 179: 1-9, 1979). While complex and a bit difficult, it all worked well and was well received. Later we used sheet film instead of individual cover slips (Neurosci Lett 25:101-105, 1981).
At this point in time in the field there were many jealousies and conflicts. There was a flap that we did not reference Roth’s contribution when we published our work. But, Roth was referenced in every draft and in the final version of the paper. Anyone who looked would have seen that. It is surprising that even bright and accomplished people can be sociopathic and hurt others quite badly. Those careless accusations created difficulties for many people for a long time. In that regard, it was not a pleasant time. But scientifically, it was exciting. We published dozens of papers showing the anatomical distribution of receptors in brain and in other tissues.
This receptor autoradiography work was a foundation of my career at that time. We helped many other labs in establishing the techniques, and freely gave out our “secrets” (for example, procedures for washing the slides, availability of condensers, etc.) to Miles Herkenham, Tom Rainbow, Pat Goldman and others. Many of these colleagues went on to make strong contributions of their own. Today, it is gratifying to see these techniques used as standard approaches in receptor studies.
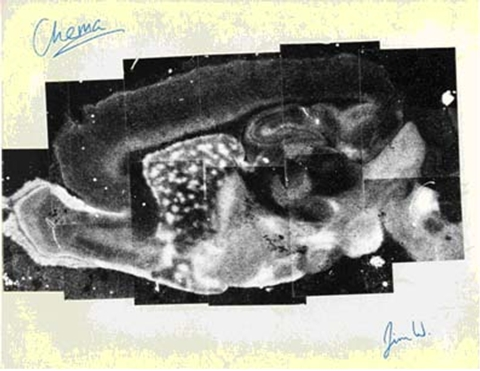
An image of the distribution of opiate receptors in a saggital section of rat brain. In vitro labeling of a slide mounted section was used. The figure is a collage of many smaller photos. This collage was given to me by two postdocs who have signed it. “Chema” is Jose Palacios, and “Jim W” is Jim Wamsley. Both fellows have had wonderful careers in academia and industry.
Image analysis of autoradiographic images with desktop computers
There is one last piece to this story, and that is the development of techniques to analyze the images. In order to quantify the film images, computers with scanning devices were used. Also, images were color coded by the computer programs so that differences in density levels could be seen more easily (Palacios et al, Neurosci Lett 25:101-105, 1981). But at that time, only large cumbersome devices existed. For example, one was at the NIH, and we did use it one time. So we set about trying to develop equipment whereby images could be quantified and analyzed on desk top (PCs) computers.
Peter Whitehouse, a very knowledgeable and alert fellow, put us in contact with Harry Loats and his colleagues, who had exactly the skills needed to do this. They developed and improved the equipment and software and it was tested in my lab. In the end, we had a marketable “image analysis station” at a reasonable price. Competition developed quickly and such software and hardware now exist throughout the world.
Who did what?
Human interest stories about scientists can be fascinating. James Watson’s “Double Helix” was quite popular. Sometimes scientists, being human, forget who did what and how things were done. In a book called Molecules of Emotion (1997, Scribner, NY) the author confuses the order in which things were done and who did the developmental work in receptor autoradiography. In fact, the home department of the senior developer was not even correct in the book. The autoradiographic approach to localizing receptors in brain was first developed by Mike Kuhar, a collaborator (H Yamamura), and a graduate student (W Scott Young III) who are the authors of the relevant papers. Existing notebooks, photography logs, dates of publications, and written recollections of those involved solidly support this. The appropriate publications are: Kuhar, M.J. and Yamamura, H.I. Light Autoradiographic Localization of Cholinergic Muscarinic Receptors in Rat Brain by Specific Binding of a Potent Antagonist. Nature 253: 560‑561, 1975, and Young, W.S., III, and Kuhar, M.J. A New Method for Receptor Autoradiography: [3H]Opioid Receptors in Rat Brain. Brain Res. 179: 255‑273, 1979. Collaborations with others began after the details were worked out by this initial group.